Table of Contents
Hypotonic vs Hypertonic vs Isotonic
Before you delve into the differences between hypotonic and hypertonic, you need to understand the origin of these terms and why you should know about them. The concept of hypotonic and hypertonic is associated with all the energy drinks or other soda-based fluids we frequently guzzle down without a thought.
These two terms have their roots in osmosis—the movement of water molecules from a higher to a lower concentration through a semipermeable membrane. This cell membrane allows only the solvent particles to pass through it.
A certain level of pressure must be applied to a solution to prevent fluid movement when the solution is being separated from pure water. The measure of this pressure is called tonicity. Tonicity exists in three states—isotonic, hypotonic, and hypertonic.
This guide will focus primarily on hypertonic and hypotonic states. So, let’s get started on the discussion!
What Happens in the Hypertonic State?
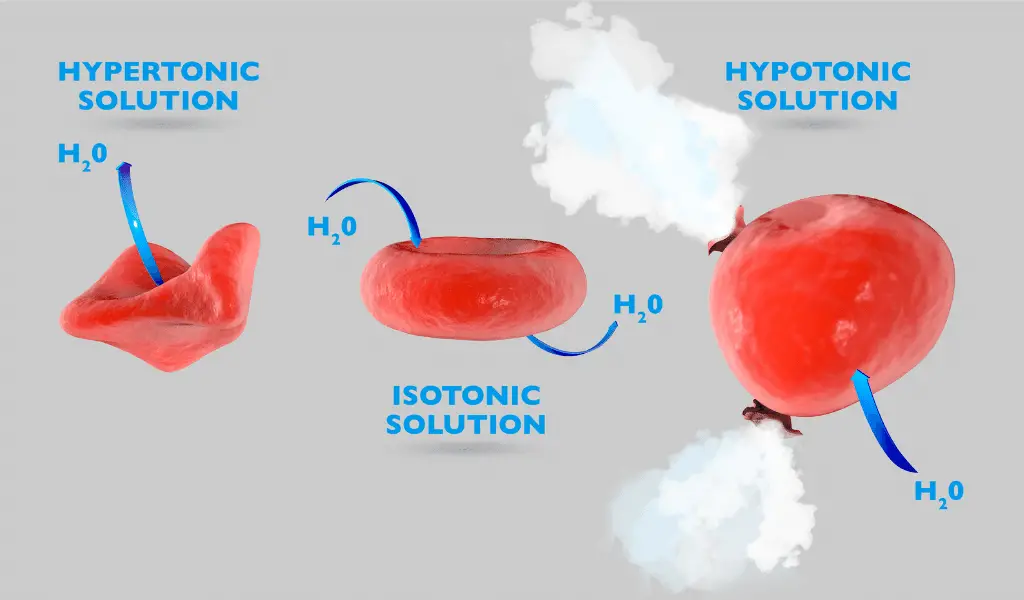
A solution is in a hypertonic state when it has a very high solute concentration compared to the inside of the cell. Due to this, the osmotic pressure of the solution is relatively high. Osmotic pressure is the force that prevents the movement of water.
When a cell is suspended in a hypertonic solution, water molecules will move out of the cell and blend into the solution due to the osmotic water potential. As water moves to the outside of the cell, the cell gets wrinkled and distorted. This effect is called ‘crenation.’
In the case of plants, the plasma membrane detaches from the rigid walls of the cell but remains joined to the wall at specific points. This detachment happens due to crenation and eventually leads to ‘plasmolysis.’
Importance of Hypertonic Solutions
Suppose you look at the world of sports drinks. In that case, hypertonic solutions contain a higher concentration of sugar and salt than blood. They easily supplement your regular carb intake and increase glycogen storage.
Among all energy/sports drinks, hypertonic drinks provide the highest dose of carbohydrates (>8%). Hypertonic solutions are also helpful for preserving food. For instance, if you dip a packet of fruits or fish in a hypertonic salt solution, it kills the microbes breeding inside the package.

This is because the water content in microbial cells is high than the level of water present in hypertonic solutions. As a result of the concentration gradient, water can flow out of cells. The lack of water causes the cells to shrink and eventually kill microbes.
What Happens in the Hypotonic State?
In the hypotonic state, water molecules in the solution tend to get inside the cell due to the osmotic pressure. This can make the cells swell, and if the internal pressure increases, they might burst. A hypotonic solution has a lower solute concentration compared to other solutions.
In the plant cells, hypotonic solutions may lead to turgidity. Due to the swelling of the cell, the membrane gets attached to the cell wall. As the wall is rigid, it prevents the cell from bursting. This effect is known as turgidity or cytolysis, and we call the swollen cell a ‘turgid cell.’
Importance of Hypotonic Solutions
Hypotonic solutions have a relatively lower concentration of salt and carbohydrates (<5%). This makes them easier to get absorbed into the bloodstream. Faster absorption ensures quick hydration and a speedier release of electrolytes.
When you consume a hypotonic drink, the gut lining quickly absorbs it, replacing the fluid loss. So, the risk of cramps, bloating, or GI distress is much lower than other solutions.
Similarities Between The Hypotonic and Hypertonic Solutions:
- Both hypotonic and hypertonic extracellular fluids are described in terms of osmolarity.
- In both states, there are solvent molecules and solute molecules.
- In either case, there is a net movement of solvent molecules.
Differences Between Hypotonic and Hypertonic Solutions:
| Hypotonic Solutions | Characteristics | Hypertonic Solutions |
| Fewer solutes and more solvents | Water Concentration | Higher concentration of solutes and fewer solvents |
| Push water into the cell, causing swelling | Movement | Pulls water out of the cell, causing the cell to shrink |
| Lower osmotic pressure | Pressure | Higher osmotic pressure |
| Not used as a preservative | Use | Can act as a preservative by killing microbes |
| Quickly replaces water loss in our bodies (ex. sports drinks) | Benefit | Can help treat cerebral hemorrhages |
Check out the quick guide below to get a better overview of the differences between the two types of solutions:
| Hypertonic | vs. | Hypotonic |
| ‘Hyper’ means high, and ‘tonic’ refers to fluid. So, it means a solution with a high solute concentration. | Definition | ‘Hypotonic’ means a fluid that has a low concentration of solute. |
| Low amount of water | Water Concentration | High amount of water |
| Molecules are pushed out of the cell. | Movement of Particles | Molecules move into the cell. |
| The cell shrinks. | Effect on Cell | The cell swells. |
| Plasmolysis may occur in plant cells. | Impact on plant cells | Cytolysis may occur in plant cells. |
| Quickly restores glycogen stores. | Positive impacts on the body | Regulates muscle function and balances fluid levels with a lower risk of bloating. |
| Easily dehydrates the body and might cause nausea. | Negative impacts on the body | Provides less energy and can prove useless after a long run. |
Final Thoughts
The concepts of hypotonic and hypertonic solutions are essential for health practitioners. But it’s also crucial for the common public to avoid emergency cases like severe dehydration, hemorrhage, and increased sodium in the blood.
The above guide simplifies the idea of tonicity and explains how water molecules behave in these two states while also shedding light on the differences between the two.