An element is a substance made of a single type of atom, while a compound is a chemical blend of different elements.
Elements and compounds are two substances that make up the world in which we live. Every object found on this Earth is made of one element or multiple elements, which are called compounds.
Table of Contents
What is an Element?
An element is a pure chemical substance. It contains a single type of atom, and it can’t be broken down further by any type of chemical reaction.
The Periodic Table of the Elements
The periodic table organizes the different types of elements. Each atom in the element has the same atomic number in the periodic table, and elements are represented using symbols and numbers.
The atomic number indicates the number of protons.
There are approximately 118 elements known at present on Earth. Out of these, 94 occur naturally.
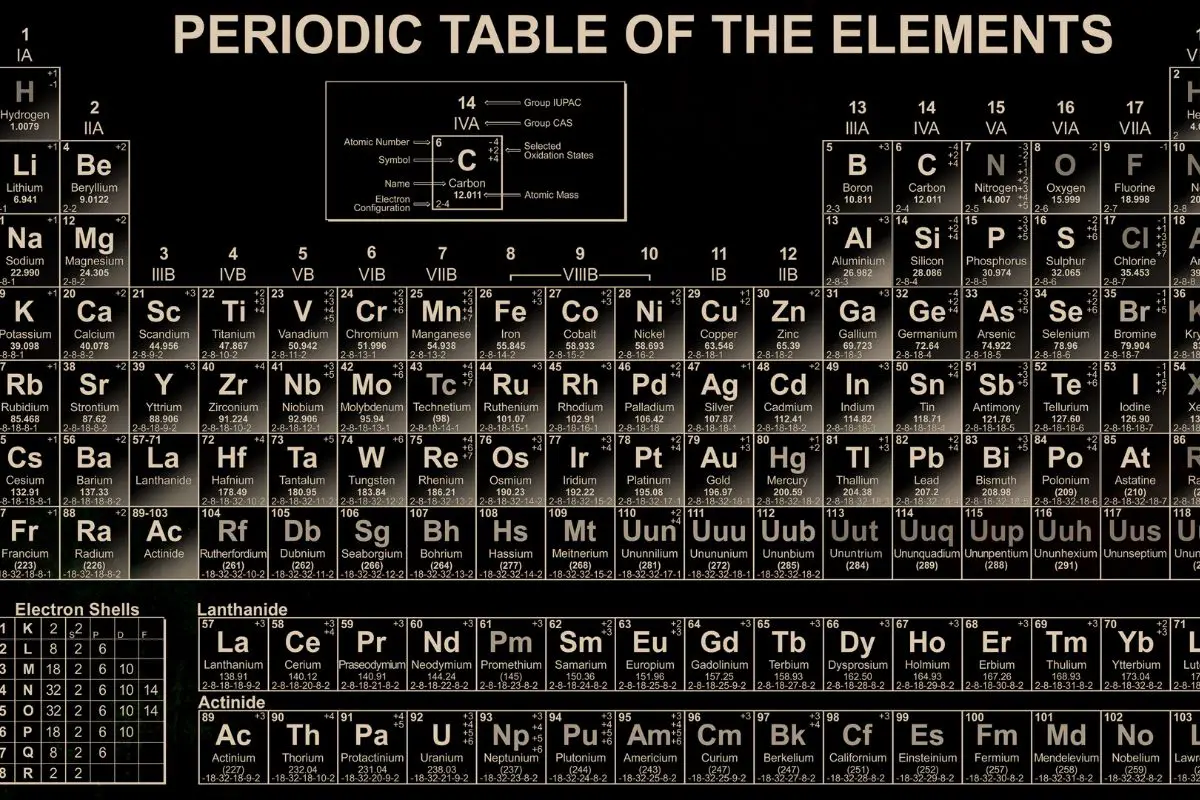
Sometimes two or more types of atoms can have the same atomic number and position in the periodic table. This is called an isotope.
Examples of Elements
Elements are categorized into three types: metals, nonmetals, and metalloids.
Metals
Metals are present on the left side of the periodic table. They possess strong electro-conductivity and magnetic properties. Other aspects of metals include high malleability, ductility, and melting point, for example.
Examples: Copper, aluminum, gold, iron, and mercury
Nonmetals
You’ll find the nonmetals on the right side of the periodic table. Nonmetals lack the properties metals possess, are not malleable, magnetic, electro-conductive, and have a low melting point.
Examples: Oxygen and carbon
Metalloids
Elements that lie between metal and nonmetals are called metalloids. There are only seven metalloids present, and they have mixed properties of both metals and nonmetals.
For example, some metalloids can conduct electricity but only at specific temperatures. These metalloids are called semiconductors.
Examples: Silicon and arsenic
What is a Compound?
Compounds, unlike elements, are substances formed when two or more different elements are combined in a definite proportion. A chemical bond fixes the elements in the compound.
During chemical bonding, the elements lose some of their properties, and the newly formed compound has a new property. This results in a compound that can be broken down into simpler substances by chemical reaction and is usually represented by its chemical formula.
There can be an endless number of compounds formed by combining different elements.
For example, NaCl is formed when Sodium (Na) and chloride (Cl) come together and form an ionic bond.
Types of Compounds
Compounds are categorized based on bond formation. Some types are molecular compounds, ionic compounds, intermetallic compounds, and complex compounds.
- Covalent bonds form molecular compounds.
- Ionic bonds form ionic compounds.
- Metallic bonds form intermetallic compounds.
- Coordinate-covalent bonds form complex compounds.
What is the Difference Between an Element and a Compound?
| Element | vs | Compound |
| 118 known, 94 naturally occurring | Total Number | An endless number of compounds can be formed |
| Metals, nonmetals, and metalloids | Types | Molecular compounds, ionic compounds, intermetallic compounds, and complex compounds |
| Represented using symbols and numbers | Representation | Represented by their chemical formula |
| Elements can be distinguished by their atomic number | Distinguished By | Compounds can be distinguished by the definite ratio of elements combined to form different compounds |
| Contain a single type of atom. All the properties of that atom are represented by that element | Composition and Property | Made of two or more elements in definite proportion through chemical bonds |
| Cannot be broken down by any chemical reaction | Ability to Breakdown | Can be broken down into simpler substances by chemical reactions. |
Explore the Differences Between Elements and Compounds
Elements and compounds are two essential parts of physical science, and the whole of chemistry revolves around them. The main difference is that an element contains a single type of atom, and compounds are substances formed from two or more elements.
If you’ve enjoyed learning about the difference between an element and a compound, check out our post about the differences between solutes and solvents.